
Theoretical modelling of the physisorption (i.e. The mass percentage seems too small for being attractive in mobile applications (although the early company Zevco in the United Kingdom proposed the concept in connection with its alkaline fuel cell taxi prototype).

The advantage of raising pressures to near 30 MPa has been considered ( Ahluwalia et al., 2010).Ĭompared with storage in liquid hydrogen, the difference between the 20 and 77 K temperature should reduce filling costs and reduce problems with escaping hydrogen. A value of 1.5% is established in practice ( Nijkamp et al., 2001 Züttel, 2004). The maximum specific surface area of carbon structures is 1 312 m 2 kg − 1. The maximum amount of hydrogen adsorbed is thus directly proportional to the effective carbon surface area, and for thin sheets of graphene, activated carbon, or graphite, the proportionality factor is about 2% by mass, i.e.

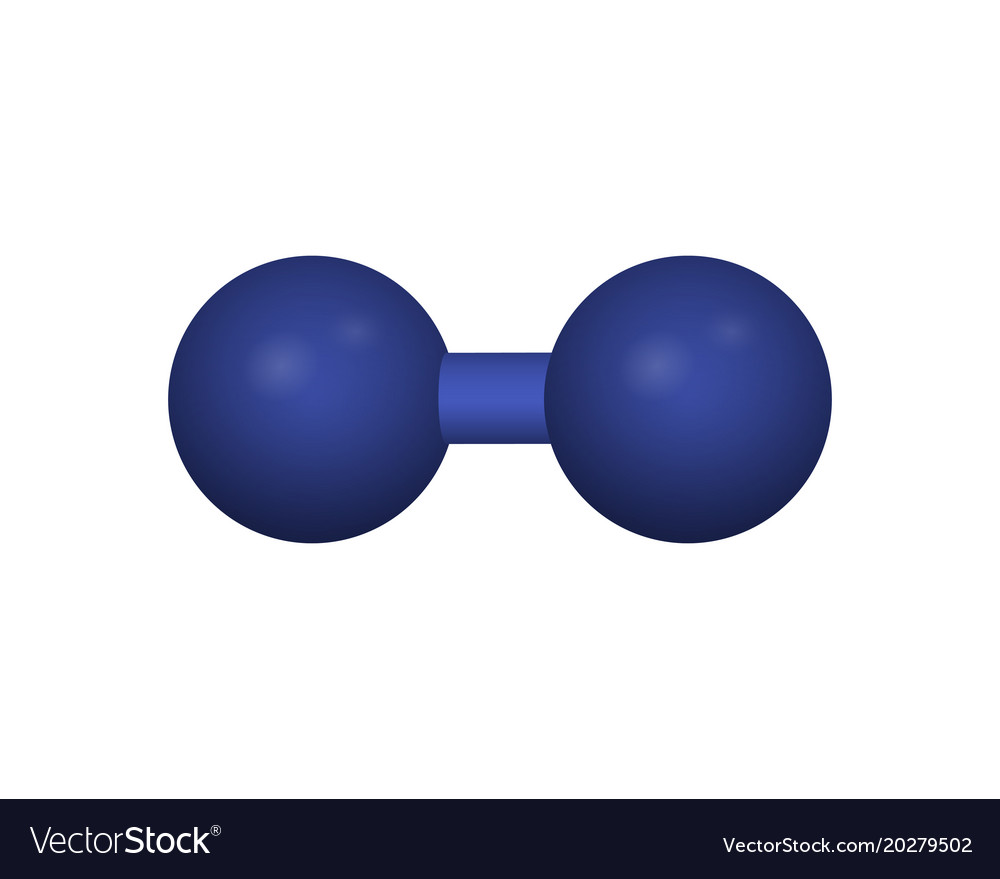
For a fully occupied monolayer, the hydrogen concentration may be 1.2 × 10 − 5 mol m − 2. The effective surface area can be up to thousand times larger than the geometrical area. This makes activated carbon, a material with a high number of micropores that can be created by pyrolysing the carbon together with inert gases (such as argon) at an elevated temperature, highly suitable. Because only a monolayer is formed, the effective surface area must be as large as possible in order to get interesting storage parameters such as hydrogen mass or volume fraction. Too high a temperature leads to losses of adsorbed molecules by thermal disturbance, and for the only substance studied extensively so far, carbon, suitable temperatures are around the boiling point of nitrogen (77 K) and at pressures of about 10 MPa. Adsorption of hydrogen takes place some 0.1 nm above the surface, in a single (mono) layer at a suitable low temperature (but above the hydrogen liquid phase transition temperature of 20 K). Hydrogen molecules may adsorb not only to metal surfaces but also to surfaces of various solid materials such as carbon, zeolites, alumina, and silica, qualitatively following an adsorption and/or chemisorption curve of the type shown in Fig. Bent Sørensen, Giuseppe Spazzafumo, in Hydrogen and Fuel Cells (Third Edition), 2018 2.3.4 Cryo-adsorbed gas storage


 0 kommentar(er)
0 kommentar(er)
